Mechanism of Action
P128 has a novel, receptor‑mediated mechanism and has shown strong activity in vitro and in vivo against multidrug‑resistant Staphylococcus aureus, including MRSA, VRSA and daptomycin‑resistant strains.
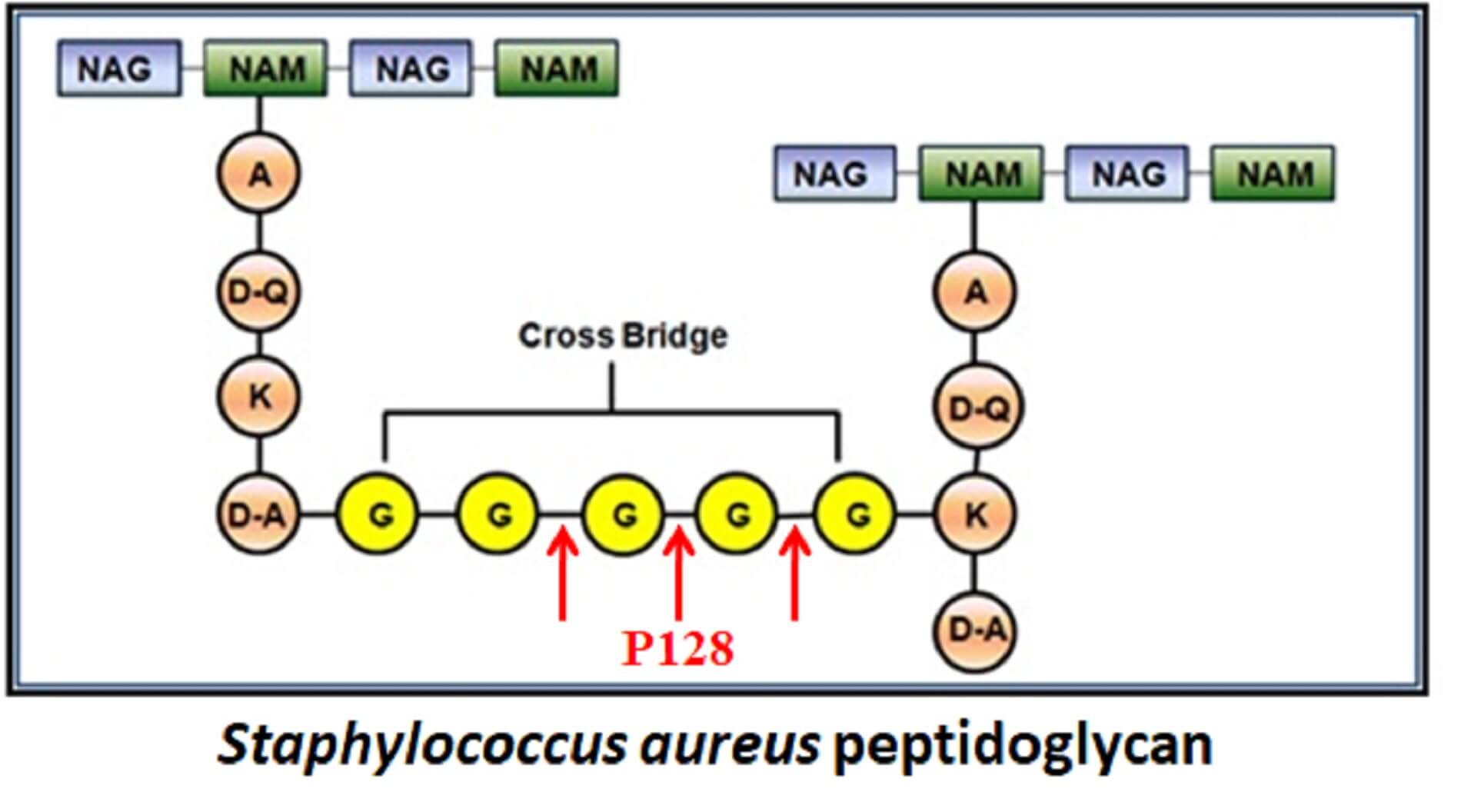
P128 is GangaGen’s lead ectolysin, a recombinant chimeric protein with two distinct functional domains. The catalytic domain is a muralytic enzyme, while the binding domain targets staphylococcal cell‑wall components, enabling highly specific recognition of Staphylococcus. As an endopeptidase, P128 cleaves key peptide linkages in the peptidoglycan, causing rapid degradation of the cell wall, osmotic lysis and death of both coagulase‑positive and coagulase‑negative Staphylococci.

P128 has a novel, receptor‑mediated mechanism and has shown strong activity in vitro and in vivo against multidrug‑resistant Staphylococcus aureus, including MRSA, VRSA and daptomycin‑resistant strains.
GangaGen has developed a scalable manufacturing process to produce P128 at clinical and commercial scale while meeting regulatory quality standards for biologics. A topical hydrogel formulation has been evaluated for nasal decolonization and skin applications in preclinical and clinical studies.
P128 has progressed into human clinical trials for decolonization of S. aureus, including MRSA, and is being developed as a therapy for serious staphylococcal infections such as bacteremia.
GBPL is also advancing Ectolysin P128 for intravenous treatment of bacteremia caused by Staphylococcus Species. P128 has progressed into human clinical trials for decolonization of S. aureus, including MRSA, and is being developed as a therapy for serious staphylococcal infections such as bacteremia.